

These positive particles made up the other half of the atomic world that the chemists had already worked out. Protons could also be observed directly as well as ions as "anode" rays. His discovery was a byproduct of studying electrons. Electrons could also produce something else. It was quickly learned that electrons could be formed into beams and manipulated into images that would ultimately become television. These were called cathode rays because they came from the cathode, or negative electrode, of these discharge tubes. Thompson was the first to observe and understand the small particles called electrons. Other scientists studying the discharge effects of electricity in gasses made some direct discoveries. The holes in that knowledge were filled by inference, conjecture and discovery. A lot was learned by logic and reason and correctly assembling previous knowledge. This business of filling in the holes has continued today as new elements are added to the end of the table every few years. The other big thing that the chart did was to help chemists predict the elements that they had not found. The weights of the elements were found to increase as the elements advanced through the periodic chart and there seemed to be a number, called the atomic number that explained the regular chemical properties. Scientists made numerous conjectures and some theoretical predictions about what the nature of atomic structure really was. The regularity of the table and the observed combinations of chemical compounds prompted some scientists to infer that atoms had regular repeating properties and that maybe they had similar structures. The great Periodic Table of Elements by Mendeleyev gave scientists two very important things. Scientists in the 1800's were able to infer a lot about the sub-atomic world from chemistry.
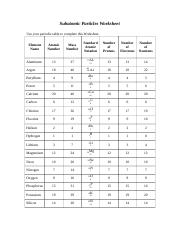
They are direct observation, indirect observation or inferred presence and predictions from theory or conjecture. There are three ways that scientists have proved that these sub-atomic particles exist.

If there is no way in the world to see an atom, then how do we know that the atom is made of protons, electrons, neutrons, the nucleus and the electron cloud?


 0 kommentar(er)
0 kommentar(er)
